- Patients are looking for procedures that help them improve their quality of life and return to daily activities1
- Efficient use of hospital resources is becoming increasingly important in the management of patients post-aortic valve replacement (AVR)2,3
- Compared with surgery (sAVR), TAVI reduces hospital stay length, increases discharge straight to home, shortens procedure time and reduces rehospitalisation4
Patient and hospital goals for aortic valve replacement
Following AVR, patients want to avoid complications, return home and re-engage with their daily lives.1 Early discharge can help to improve patient outcomes, quality of life and conserve hospital resources.5
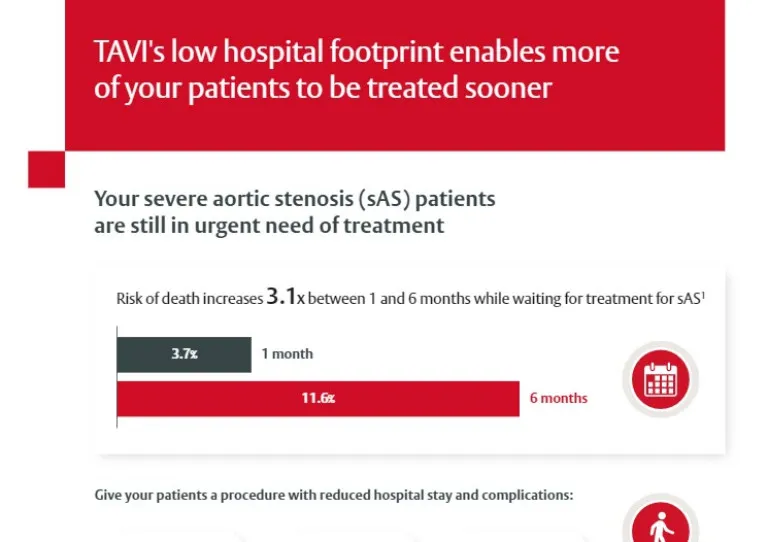
Can TAVI allow us to more easily achieve those goals?
As well as superior* clinical outcomes (TAVI compared with sAVR), data from the PARTNER 3 Trial showed:4
- A 4-day shorter length of hospital stay
- A higher proportion of patients discharged to home or self-care
- Less complications leading to rehospitalisation
- A 1-day shorter length of stay in intensive care
- A shorter procedure time by over 2 hours
*PARTNER 3 Trial proved SAPIEN 3 TAVI is superior to surgery for the primary endpoint and multiple pre-specified secondary endpoints.
Proven benefits of TAVI over sAVR across the patient and healthcare pathways
Fewer complications, shorter procedure times and reduced hospital stays can help to reduce the burden on limited healthcare resources.4
 For further details, explore the full PARTNER 3 Trial
For further details, explore the full PARTNER 3 Trial
Rapid discharge following TAVI demonstrated in real-world studies
Data from 3M TAVR and FAST-TAVI, two real-world studies looking at optimising patient discharge pathways following TAVI, showed:3,6

Early discharge to home can help to improve patient quality of life, conserve hospital resources and reduce treatment costs.
See details on the quality of life benefits of TAVI† Composite of all-cause mortality or stroke by 30 days.
‡ Composite of all-cause mortality, vascular access-related complications, permanent pacemaker implantation, stroke, re-hospitalisation due to cardiac reasons, kidney failure, and major bleeding at 30 days.
References:
1 Coylewright M, et al. Health Expect. 2016;19(5):1036-1043.
2 Arora S, et al. Circ Cardiovasc Interv. 2018;11(9):e006929. doi:10.1161/ CIRCINTERVENTIONS. 118.006929.
3 Barbanti M, et al. Eurointervention 2019;5:147-154.
4 Mack M et al. N Engl J Med 2019;380:1695-1705 and supplementary material.
5 Barbanti M, et al. Heart. 2015;101:1485–1490.
6 Wood D, et al. JACC: Cardiovascular Interventions 2019;12(5):459-469.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-0776 v2.0