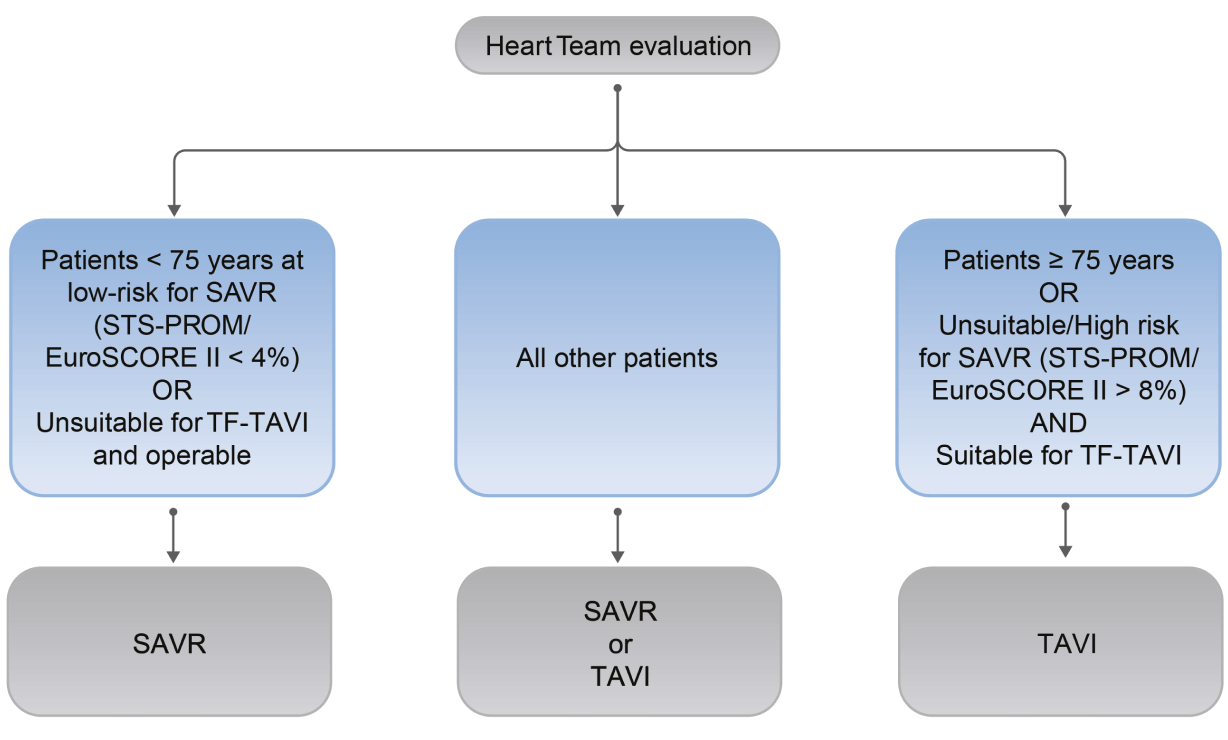
Referral and treatment recommendations change for sAS patients
With the updated 2021 ESC/EACTS Guidelines for the management of valvular heart disease (VHD), the referral and treatment decisions for patients with severe aortic stenosis (sAS) have changed:1
- All severe aortic stenosis (sAS) patients* should now be evaluated by the Heart Team1
- The guidelines recommend TF-TAVI for as the preferred mode of intervention in patients ≥75 years of age**, as well as for additional patient groups <75 years of age1
- The Heart Team recommendation should be discussed with the patient, who can then make an informed treatment decision between the option of TAVI or SAVR1
*With treatment indication.
**Based on evaluation of clinical, anatomical and procedural factors.
2021 ESC/EACTS VHD Guidelines: Treatment Recommendations for sAS patients
Full guidelines details can be found on the official ESC website, you can also preorder a free pocket guidelines below.
The future of TAVI - Edwards Lifesciences satellite symposium
Listen to leading experts discuss the impact of the updated 2021 ESC/EACTS VHD Guidelines on ESC Congress – The Digital Experience 2021
The Future of TAVI, ESC 2021 - 44 min
- Introduction, purpose and learning objectives by Professor Bernard Prendergast (00:38)
- An overview of the 2021 ESC/EACTS Guideline recommendations for the treatment of severe AS and the evidence supporting these changes by Professor Jan-Malte Sinning (04:40)
- The evolution of the ESC/EACTS Guidelines, the increased number of TAVI procedures, the economic impact of TAVI, and the future role of TAVI in severe AS by Dr. Francesco Saia (12:25)
- A panel discussion of the topics covered initiated by Professor Martine Gilard (22:53)
- A case study and discussion on the benefits of holistic management by Professor Jörg Kempfert (31:22)
Free ESC/EACTS Pocket Guidelines for VHD
Sign up to receive:
- 2021 ESC/EACTS Pocket Guidelines for the management of valvular
heart disease - Educational emails on Aortic Stenosis Management
- Useful resources for you and your patients
Thank you for your order
Thank you for ordering the 2021 ESC/EACTS Guidelines for the management of valvular heart disease and your interest in keeping in touch with Edwards Lifesciences.
The Pocket Guidelines hard copy will be delivered to you in few weeks’ time.
References:
1. Vahanian A, et al. Eur Heart J. 2021; ehab395. doi:10.1093/eurheartj/ehab395.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-2675 v4.0