Today, 80 is the new 701
And patients in their 80s deserve a treatment that can help keep them moving forward
When patients are faced with a treatment decision, they consider more than the data. Transcatheter aortic valve implantation (TAVI) addresses the key concerns of patients treatment decisions, including: survival, reducing the fear of heart failure, improving their quality of life, and retaining their independence.2

Patients today have a life expectancy that's nearly a decade longer compared to

Patients in their 80s are less likely to be treated3
Treatment delays can have serious consequences

Nearly 40% of patients will have an
during a waiting period of 4 months8

11.6% mortality rate at 6 months
for those waiting for AVR†9
*As compared to surgical aortic valve replacement (SAVR).
†AVR = aortic valve replacement.
Timely referral is crucial
Transcatheter aortic valve implantation (TAVI) patients in their 70s and 80s showed comparable quality-of-life (QoL) improvements and clinical benefits1
Prior to treatment, QoL was self-reported by patients as poor. Post-treatment, patients in their 80s self-reported similar QoL improvement to patients in their 70s. After TAVI, patients in their 80s reported comparable QoL improvements to those patients in their 70s.
Offer your patients in their 80s a chance for more:1
Transcatheter aortic valve implantation (TAVI) patients in their 70s and 80s showed comparable quality-of-life (QoL) improvements and clinical benefits1
70 and 80 year old patients report similar KCCQ‡ scores before AVR1
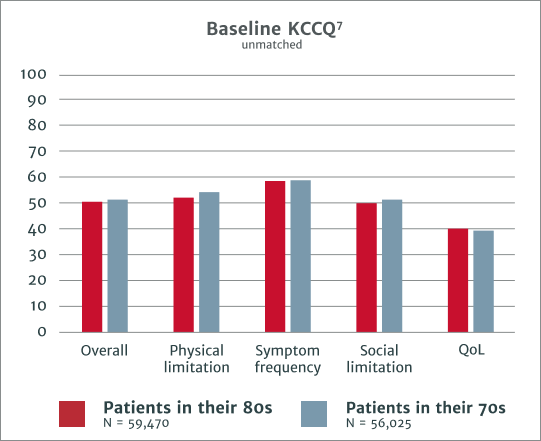
Comparable improvement and outcomes in KCCQ scores at 1 year include1
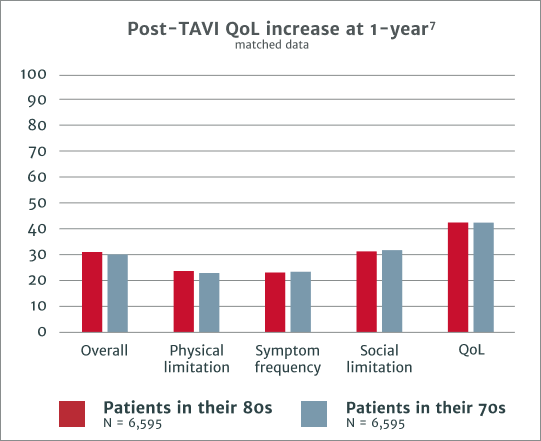
‡KCCQ = Kansas City Cardiomyopathy Questionnaire
NYHA class at 1 year7
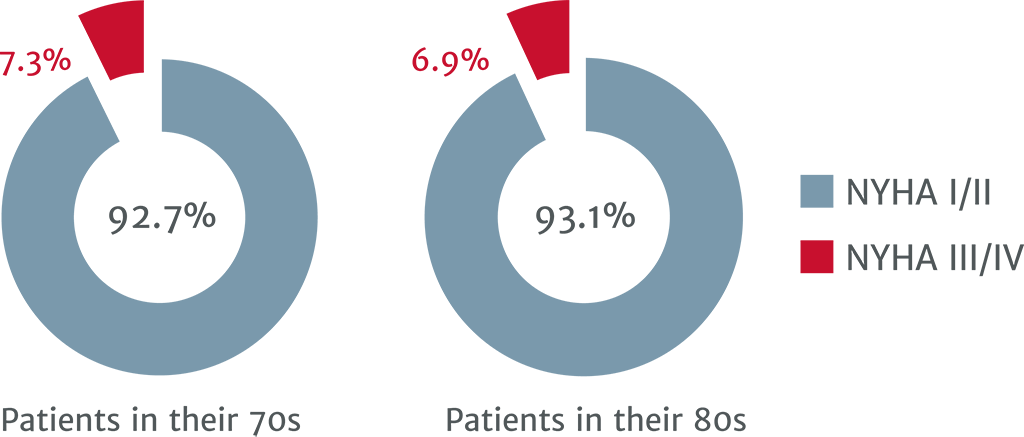
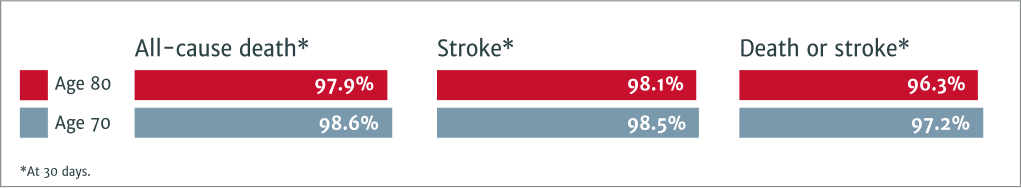
Patients in their 80s benefit from TAVI as much as patients in their 70s:
Clinical benefits include freedom from:1
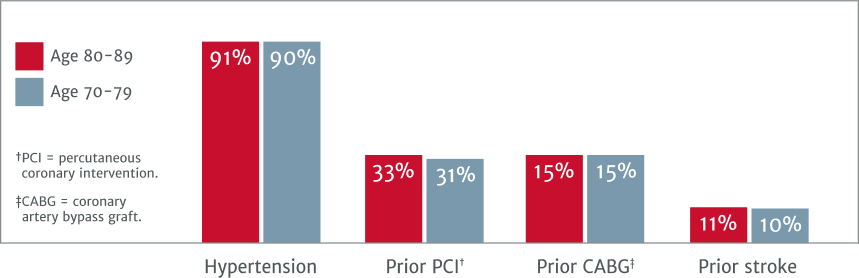
Looking at the baseline characteristics of these two patient groups, severe AS patients in their 70s and 80s present with similar clinical characteristics:
Between both TAVI patient age groups, baseline characteristics are similar:1
ESC/EACTS VHD guidelines recommend TAVI for severe AS patients aged 75+10
Timely referral is crucial - guide your 80-year-old SAS patients to a heart team evaluation.
References:
1 Data on file. Edwards Lifesciences, 2022.
2 Col NF, Otero D, Lindman BR, Horne A, Levack MM, Ngo L, et al. (2022) What matters most to patients with severe aortic stenosis when choosing treatment? Framing the conversation for shared decision making. PLoS ONE 17(8):e0270209. https://doi.org/10.1371/journal.pone.0270209.
3 Eugène M, et al. Contemporary Management of Severe Symptomatic Aortic Stenosis. J Am Coll Cardiol. 2021 Nov 30;78(22):2131-2143.
4 Eurostat. Life expectancy at birth 2021. Accessed August 8, 2023.https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Table01_Life_expectancy_at_birth_2021.png.
5 OnS.gov. Office for National Statistics. https://www.ons.gov.uk/.
6 Strange GA, Stewart S, Curzen N, et al. Uncovering the treatable burden of severe aortic stenosis in the UK. Open Heart 2022;9:e001783. doi:10.1136/openhrt-2021-001783.
7 Data on file, Edwards Lifesciences.
8 Elbaz-Greener G, Yarranton B, Qui F, et al. Association between wait time for transcatheter aortic valve replacement and early postprocedural outcomes. J Am Heart Assoc. 2019;8(1):e010407.
9 Malaisrie SC, McDonald E, Kruse J, et al. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98(5):1564–1570.
10 Vahcinicin A, et. al. ESC/EACTS Scie11tific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Hear J. 2022 Feb 12;43(7):561-632. doi: 10.1093/eurheartj/ehab395. Erratum in: Eur Heart J. 2022 Feb 18;: PMID: 34453165.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-7401 v2.0