Resources
Access a wealth of free resources for use in your clinical practice and supporting information for your patients. The materials come in many different formats from downloads and videos, to physical leaflets and models. All physical resources are currently available only for health care professionals in Europe, except France. Search below to find out more.
Filters:

2021 ESC/EACTS Guidelines for Valvular Heart Disease
Receive your free ESC/EACTS Pocket Guidelines for VHD

Severe aortic stenosis diagnosis and treatment
A general cardiologist’s guide to support female patient diagnosis

Lit review on elderly population
A summary of literature around elderly population and TAVI
Edwards TAVI study compendium
In-depth summary of the key Edwards Lifescience TAVI studies.

Aortic stenosis overview
Summarises the aetiology of aortic stenosis and its clinical evaluation, including the ESC/EACTS guidelines and treatment practices.

ESC/EACTS 2021 AS guideline flowchart
Graphic summary of the ESC/EACTS 2021 treatment guidelines.
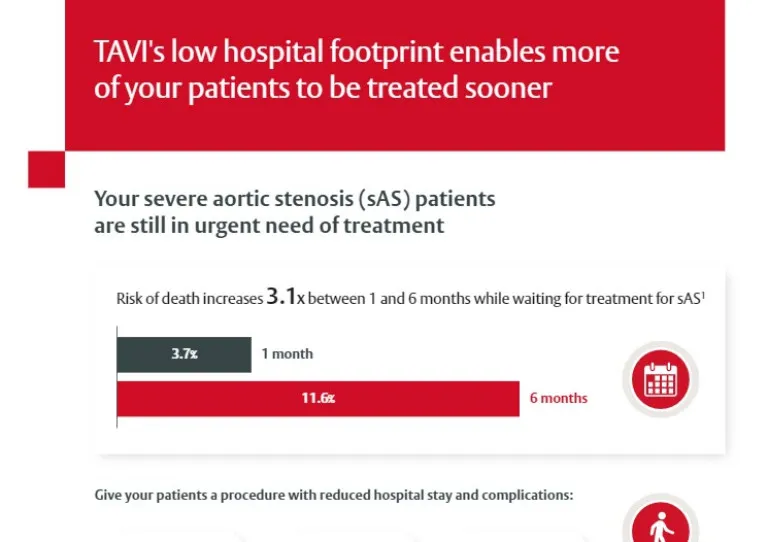
Healthcare system benefits
A summary of the impact of TAVI on the healthcare system and hospital resources.

Optimising echocardiography
Optimising echocardiography to support aortic stenosis assessment and severity grading

Severe aortic stenosis diagnosis and treatment
A general cardiologist’s guide to support female patient diagnosis

What is aortic stenosis? - Brochure for patients
A patient guide that explains the impact of Aortic Stenosis on their life and heart.

Tips for caregivers
A summary of helpful tips for carers and families to support patients living with aortic stenosis.

Aortic stenosis symptom tracker
A patient diary to assist in recording and monitoring symptoms, which can be brought along to future clinical consultations.
Shared Decision Making patient brochure
A guide for patient to take ownership of their treatment decision.

Treatment options Brochure
A patient guide that explains the differences between TAVI and surgical valve replacement.

What is TAVI? - Brochure For patients
A patient guide that explains the TAVI procedure and its benefits

TAVI with the Edwards SAPIEN 3 platform
Several studies have shown that transcatheter aortic valve implantation (TAVI) has benefits for patient symptoms, recovery and quality of life compared with surgical aortic valve implantation (SAVR).
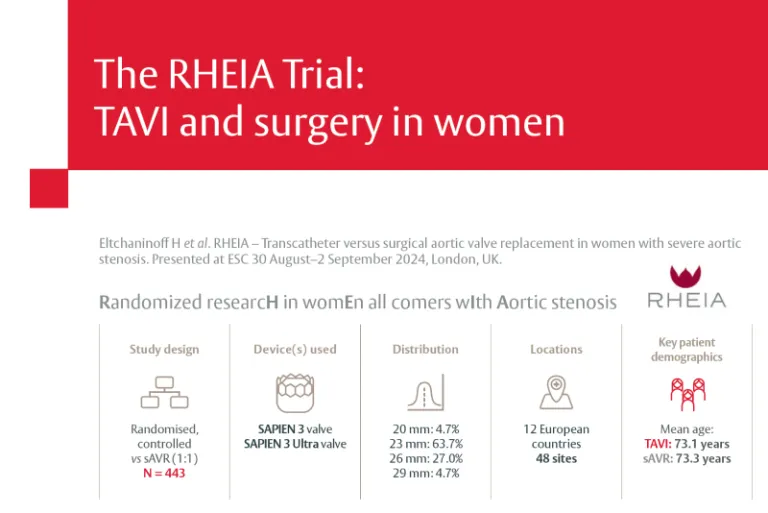
The RHEIA Trial: TAVI and surgery in women
The world’s first women-only randomised clinical trial comparing TAVI with sAVR
No results found
Remove filters and search again.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP–EU-0778 v2.0