- Around three-quarters of severe aortic stenosis (sAS) patients are believed to be undiagnosed1
- Early detection and timely referral of these patients is key to reducing mortality and morbidity2,3
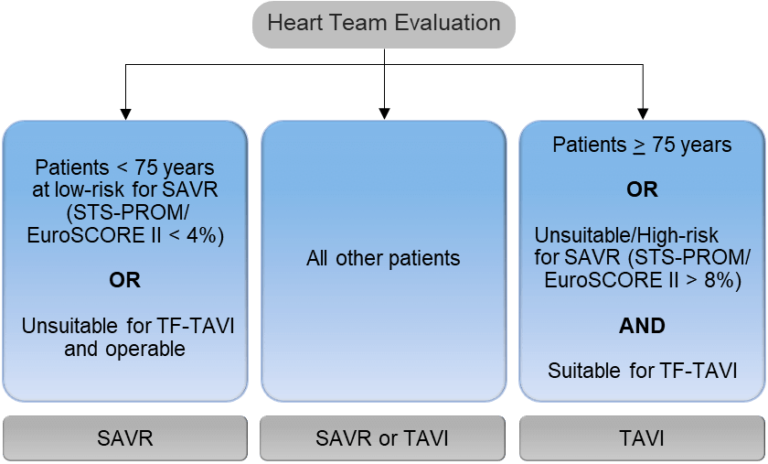
- 2021 ESC/EACTS VHD Guidelines now recommend that all patients of any age with sAS* are referred to the Heart Team4
*With a treatment indication
Severe aortic stenosis prevalence and prognosis
- The frequency of aortic stenosis (AS) cases increases with advancing age many of whom remain undiagnosed in part due to a low level of symptom awareness2
- Without valve replacement the future for sAS patients is bleak, with survival rates as low as 50% at 2 years and 20% at 5 years5
Estimated number of sAS patients in the European Union by age2

Defining and assessing aortic stenosis severity
- Accurate assessment of AS severity is key for optimal therapeutic decision making6
- The 2021 ESC/EACTS Guidelines:4
— Provide a definition of severe aortic stenosis
— Advocate echocardiography as the key diagnostic tool
— Recommend using a step-by-step and integrated approach for severity grading
2021 ESC/EACTS stepwise integrated approach for the assessment of aortic stenosis severity4
High gradient aortic stenosis
In patients present with high gradient AS, echocardiography alone is sufficient in determining severity of AS. Patients will require aortic valve replacement (AVR) if presenting with symptoms and/or left ventricular (LV) systolic dysfunction.6
Low gradient aortic stenosis
- Around 40% of AS patients present with low gradient AS6
- The actual severity of low gradient AS can be difficult to assess and will often require multiple assessment modalities6
- Among patients with low-gradient AS, a substantial proportion (30% to 70%) have true sAS and may benefit from AVR6
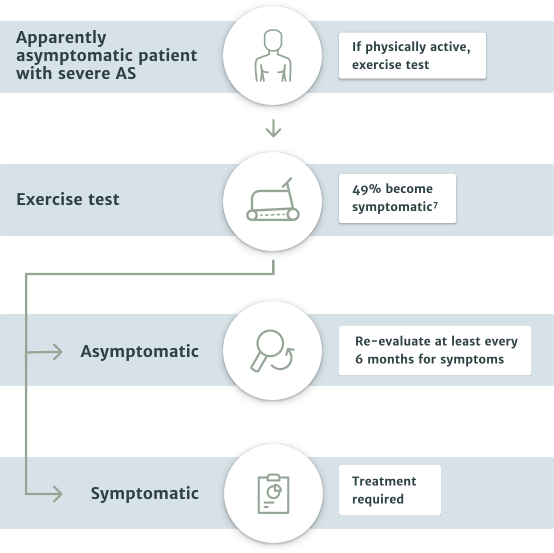
Asymptomatic severe aortic stenosis patients
- The majority of asymptomatic patients should be put on watchful waiting and periodically re-evaluated. However, in special cases some patients may be referred to the Heart Team for treatment of their aortic stenosis4
- Patients with LVEF <50% are recommended for intervention4
- In patients with LVEF >50% who remain physically active, an exercise test is recommended to identify those with abnormal results, such as the appearance of symptoms related to aortic stenosis or a decrease in blood pressure below baseline4
- In asymptomatic patients who are unsuitable for exercise testing, there are special cases where referral to the Heart Team is recommended. This includes patients with the following risk factors:4
— systolic LV dysfunction (LVEF <55%) without another cause
— very severe aortic stenosis (mean gradient ≥60 mmHg or Vmax >5 m/s)
— severe valve calcification (ideally assessed by CCT) and Vmax progression ≥0.3 m/s/year
— markedly elevated BNP levels (>3x age- and sex-corrected normal range) confirmed by repeated measurements and without other explanation
Exercise testing is recommended for unmasking symptoms in patients who appear asymptomatic4
Related resources
Edwards TAVI study compendium
In-depth summary of the key Edwards Lifescience TAVI studies.
References:
1 Datos en archivo de Edwards.
2 Thoenes M, et al. J Thorac Dis 2018; 10:5584-5594.
3 Carabello BA. Circ Res 2013;113:179-185.
4 Baumgartner H, et al. Eur Heart J 2017; 38:2739-2791.
5 Otto CM. Heart 2000; 84:211-218.
6 Delgado V, et al. J Am Coll Cardiol Img 2019;12:267–282.
7 Généreux P, et al. J Am Coll Cardiol 2016;67:2263–2268.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-0763 v3.0