- The definitive treatment of severe symptomatic aortic stenosis (ssAS) is aortic valve replacement (AVR), either via transcatheter (TAVI) or conventional open-heart surgery (SAVR)1,2
- SAVR has been available since the 1960s and has long been the standard of care for ssAS, with over 200,000 procedures performed globally each year3
- TAVI has emerged as a less invasive approach to AVR. Since the first TAVI procedure was performed in 2002, there has been rapid growth in its use globally4
Choice of intervention for severe aortic stenosis
After referral to the Heart Team a decision to utilise SAVR or TAVI will be made. The decision is based on a number of factors, including the patient’s age, patient’s wish, estimated surgical risk, clinical characteristics, anatomical and technical aspects as well as any coexisting cardiac conditions that may require concomitant intervention.2
Treatment with either TAVI or SAVR is aimed at extending survival and enhancing patients quality of life through improvements in haemodynamic parameters.
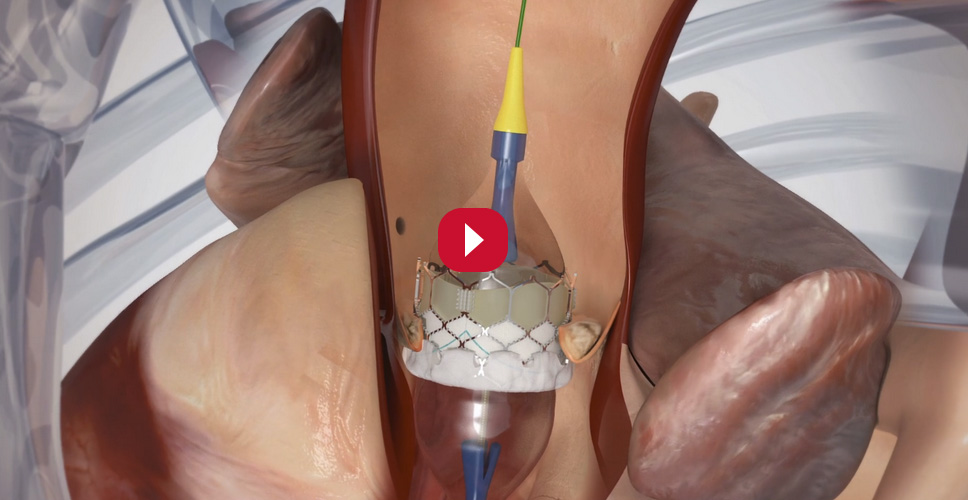
How is the TAVI procedure performed?
The TAVI procedure can be performed through several access routes, most commonly via the transfemoral approach (utilised in more than 90% of patients at most centres). This approach is possible when the patient has suitable iliofemoral anatomy and does not suffer from other vessel disease.6,7
Iterative improvements in TAVI valves and delivery systems have contributed to the increased suitability of transfemoral TAVI for patients, resulting in an increasing number of TAVI procedures.8
The SAVR or TAVI decision
The choice of intervention must be based on careful individual evaluation of technical suitability and weighing of risks and benefits of each treatment. In addition, the local expertise and outcomes data for the given intervention must be taken into account.2
Patients in whom TF-TAVI may be favourable


Patients in whom SAVR may be favourable


d Severe frailty = >2 factors according to Katz index.
e Significant multi-vessel CAD requiring surgical revascularisation, severe primary mitral valve disease, severe tricuspid valve disease, significant dilatation/aneurysm of the aortic root and/or ascending aorta, and septal hypertrophy requiring myectomy. AS, aortic stenosis; AVA, aortic valve area; BSA, body surface area; EuroSCORE, European System for Cardiac Operative Risk Evaluation; LV, left ventricular; SAVR, surgical aortic valve replacement; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; TAVI, transcatheter aortic valve implantation; TF, transfemoral.
Download full infographicThe future of symptomatic severe aortic stenosis management
The 2021 ESC/EACTS VHD Guidelines have now recommended TF-TAVI as preferred mode of intervention in patients ≥75 years of age**, as well as for additional patient groups < 75 years of age thus reinforcing this expected rise in procedures2
**Based on evaluation of clinical, anatomical and procedural factors
Find out more about the 2021 ESC/EACTS guidelines.
References:
1 Kolkailah AA, et al. Cochrane Database Syst Rev. 2019;12(12):CD013319.
2 Vahanian A, et al. Eur Heart J. 2021; ehab395. doi:10.1093/eurheartj/ehab395.
3 Cahill TJ, et al. Eur Heart J. 2018 Jul 21;39(28):2625-2634.
4 Brown JM, O’Brien SM, et al. J Thorac Cardiovasc Surg 2009;137:82–90.
5 Costa G, et al. Cardica Interv Today 2018;12(2):51-54.
6 Piazza N, Windecker S.Euro Inter 2017;12:1925-1926
7 Togweiller S, et al. JACC Cardiovasc Interv 2013;6:643-653.
8 Hamm CW, et al. Eur Heart J 2016;37:803-810.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
PP--EU-0766 v3.0